The Europe laboratory information systems (LIS) market size was valued at USD 412.21 billion in 2024 and is expected to reach USD 803.44 million by 2032, at a CAGR of 8.70 % during the forecast period
Introduction
The Europe Laboratory Information Systems (LIS) Market is evolving rapidly due to the growing emphasis on digital healthcare transformation, data-driven decision-making, and advancements in clinical laboratory automation. Laboratory Information Systems play a crucial role in managing, storing, and analyzing laboratory data, improving workflow efficiency, and ensuring compliance with healthcare regulations. Across Europe, hospitals, diagnostic centers, and research laboratories are increasingly adopting LIS platforms to streamline operations and enhance diagnostic accuracy.
The region’s strong healthcare infrastructure, coupled with supportive government initiatives promoting digital health, has significantly accelerated LIS deployment. The integration of artificial intelligence (AI), cloud computing, and advanced analytics is redefining how laboratories manage information, moving from traditional paper-based systems to intelligent, interoperable platforms capable of handling large-scale, complex datasets.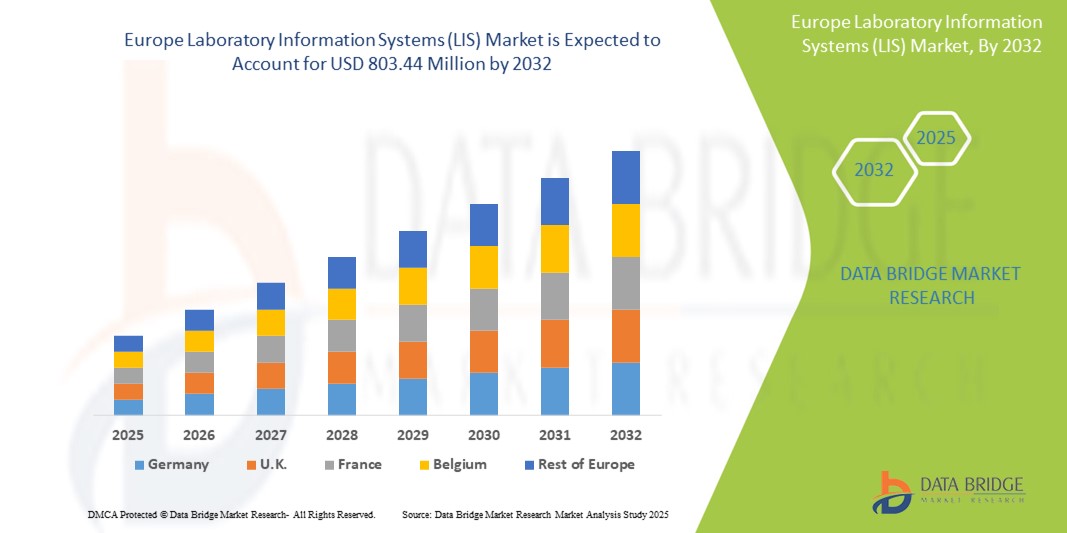
Market Overview
The Europe LIS market encompasses software and services designed to optimize laboratory processes, including sample tracking, test ordering, results management, and quality control. The market serves diverse end-users such as hospital-based laboratories, independent diagnostic centers, and pharmaceutical research organizations.
Europe’s healthcare industry has seen substantial investments in electronic health record (EHR) integration and interoperability solutions. LIS platforms are now central to these digital ecosystems, facilitating seamless communication between laboratories, clinicians, and administrative systems. The growing burden of chronic diseases, an aging population, and rising demand for accurate diagnostics further drive LIS adoption across the region.
In addition, the COVID-19 pandemic underscored the importance of laboratory efficiency and real-time data management. The need for high-throughput testing, rapid reporting, and data sharing boosted the adoption of robust LIS platforms across public and private healthcare facilities.
Key Market Drivers
Several factors are propelling the growth of the Europe LIS market. The foremost is the digitalization of healthcare systems. European countries are focusing on building interoperable healthcare networks that allow laboratories and clinical departments to exchange data securely and efficiently.
Technological advancements in laboratory automation are also key contributors. Automated analyzers, robotics, and AI-based decision support systems require sophisticated LIS software for coordination, error reduction, and data analysis. As laboratories shift towards total automation, LIS has become indispensable for integrating diverse instruments and ensuring real-time monitoring.
Moreover, stringent regulatory frameworks, such as those from the European Medicines Agency (EMA) and the General Data Protection Regulation (GDPR), have necessitated secure and compliant data management systems. LIS solutions provide audit trails, data encryption, and access control, ensuring adherence to these regulations.
Market Challenges
Despite the promising growth trajectory, the LIS market in Europe faces several challenges. One major obstacle is the high cost of implementation and maintenance. Many small and mid-sized laboratories find it financially challenging to adopt comprehensive LIS platforms due to upfront expenses, infrastructure needs, and training requirements.
Data interoperability remains another concern. Although efforts are being made to standardize healthcare data formats, integration between LIS, EHRs, and hospital information systems (HIS) is still complex. Compatibility issues among software from different vendors can lead to data silos and inefficiencies.
Additionally, cybersecurity threats are increasing as healthcare data becomes more digitized. Ensuring data privacy and preventing unauthorized access remain top priorities for laboratories and software vendors alike.
Technological Innovations
The Europe LIS market is witnessing an influx of technological innovations aimed at improving laboratory connectivity, data analytics, and decision support. Cloud-based LIS platforms have gained momentum due to their scalability, lower infrastructure costs, and remote accessibility. These systems allow laboratories to operate seamlessly across multiple locations while maintaining centralized data storage and control.
Artificial intelligence and machine learning are revolutionizing laboratory operations. LIS platforms equipped with AI capabilities can identify patterns in diagnostic data, flag abnormalities, and assist in predictive diagnostics. This not only improves test accuracy but also enhances laboratory productivity.
Another notable innovation is the integration of LIS with advanced laboratory automation tools, including robotic sample handlers and digital microscopes. The Internet of Things (IoT) enables real-time monitoring of laboratory instruments and sample conditions, reducing errors and downtime.
Market Trends
Several emerging trends are shaping the Europe LIS market:
-
Shift to Cloud and SaaS Models: Cloud-based LIS solutions are gaining prominence as laboratories seek flexible and cost-effective deployment options.
-
Interoperability and Integration: Vendors are focusing on enhancing interoperability between LIS, EHR, and laboratory automation systems to ensure unified healthcare data flow.
-
AI-Powered Decision Support: AI and analytics capabilities are being embedded into LIS platforms to aid in predictive testing and faster result interpretation.
-
Focus on Cybersecurity: With the rise in data breaches, laboratories are prioritizing secure LIS platforms with advanced encryption and compliance measures.
-
Personalized Medicine and Genomics: LIS platforms are evolving to manage genomic data, supporting personalized treatment approaches.
Competitive Landscape
The Europe LIS market is highly competitive, featuring a mix of established global players and regional vendors. Key companies focus on developing user-friendly, interoperable systems that cater to various laboratory types and regulatory requirements. Partnerships between LIS providers and healthcare organizations are common, aimed at customizing solutions to specific clinical needs.
Leading market players are investing heavily in R&D to enhance their product portfolios. Features such as mobile accessibility, AI-driven reporting, and seamless integration with diagnostic instruments are becoming standard differentiators. Additionally, mergers and acquisitions are helping companies expand their geographic reach and customer base.
European vendors often emphasize compliance with GDPR and ISO standards, positioning their products as secure and reliable. The competitive landscape also includes a growing number of startups offering niche solutions for molecular diagnostics, digital pathology, and cloud laboratory management.
Regional Insights
The Europe LIS market is geographically diverse, with varying adoption levels across countries. Western European nations, including Germany, the United Kingdom, France, and the Netherlands, lead the market due to well-established healthcare infrastructure and higher IT expenditure.
Germany represents one of the largest markets, driven by a strong focus on laboratory automation and government-led digitalization initiatives. The United Kingdom has also witnessed growing LIS implementation, particularly in the National Health Service (NHS), which aims to digitize laboratory processes nationwide.
Northern European countries such as Sweden, Denmark, and Finland are recognized for early adoption of cloud-based LIS systems and integrated healthcare solutions. In contrast, Southern and Eastern European nations are gradually catching up, with investments focused on upgrading laboratory infrastructure and ensuring compliance with EU data standards.
Future Outlook
The future of the Europe LIS market appears promising, characterized by continued technological integration, expanding application areas, and increasing demand for real-time laboratory data management. As precision medicine and genomics research advance, LIS platforms will play a central role in managing complex molecular and genetic data.
Cloud adoption will further accelerate, enabling smaller laboratories to access advanced LIS functionalities without heavy infrastructure investments. Additionally, AI-driven analytics and automation will continue to redefine laboratory workflows, supporting faster, more accurate diagnostics.
Collaborations between healthcare institutions, technology providers, and regulatory bodies will strengthen the standardization of data formats and promote interoperability across systems. Furthermore, sustainability goals are encouraging the development of energy-efficient and paperless laboratory operations, where LIS will be a foundational component.
Conclusion
The Europe Laboratory Information Systems (LIS) market stands at the forefront of healthcare digitalization, combining advanced software technology with clinical expertise to enhance diagnostic accuracy and operational efficiency. The growing emphasis on integrated healthcare ecosystems, coupled with regulatory support and technological innovation, is propelling the adoption of LIS across the region.
As laboratories evolve into data-driven diagnostic centers, LIS platforms will remain vital in enabling efficiency, compliance, and collaboration. Continuous advancements in AI, cloud computing, and interoperability will further enhance the role of LIS in Europe’s modern healthcare framework, ensuring reliable and timely diagnostic outcomes for patients.
FAQs
-
What are the key functions of a Laboratory Information System (LIS)?
-
Which European countries are leading in LIS adoption?
-
How is artificial intelligence transforming LIS capabilities?
-
What challenges do laboratories face in implementing LIS platforms?
-
How are cloud-based LIS solutions benefiting European laboratories?
Equip yourself with actionable insights and trends from our complete Europe Laboratory Information Systems (LIS) Market analysis. Download now:https://www.databridgemarketresearch.com/reports/europe-laboratory-information-system-market
Browse More Reports:
Global Electrodeionization Market
Global Electro-Medical Devices in Alzheimer’s Treatment Market
Global Electronic Filters Market
Global Electrosurgery Equipment Market
Global Electrosurgical Instruments Market
Global Emergency Shutdown Systems Market
Global Emetogenic Drugs Market
Global Emulsified Meat Market
Global Encryption Management Solutions Market
Global Endoscopic Appliers Market
Global Endoscopic Vessel Harvesting Market
Global Endosulphane Market
Global Endpoint Detection and Response Market
Global Enteric Disease Testing-Treatment Market
Global Environmental Test Equipment Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 976
Email:- [email protected]
