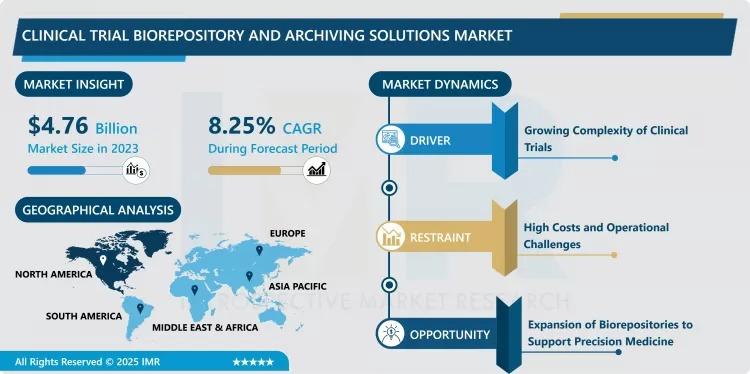
“According to a new report published by Introspective Market Research, Clinical Trial Biorepository and Archiving Solutions Market by Service Type, Application, and End User, The Global Clinical Trial Biorepository and Archiving Solutions Market Size Was Valued at USD 4.76 Billion in 2023 and is Projected to Reach USD 9.72 Billion by 2032, Growing at a CAGR of 8.25% From 2024–2032.”
The Clinical Trial Biorepository and Archiving Solutions Market plays a critical role in modern clinical research by ensuring the secure storage, management, and long-term preservation of biological samples and trial-related documentation. These solutions support regulatory compliance, data integrity, and reproducibility of clinical studies, which are essential for drug development and regulatory approvals.
Biorepository services enable the systematic handling of biospecimens such as blood, tissue, DNA, and plasma, while archiving solutions ensure safe storage of trial data, reports, and records throughout the clinical trial lifecycle. Compared to traditional storage methods, advanced biorepository and archiving solutions offer enhanced traceability, temperature-controlled environments, digital tracking, and compliance with global regulatory standards.
Growing clinical trial activities, increasing focus on personalized medicine, and stringent regulatory requirements are driving adoption across pharmaceutical companies, biotechnology firms, contract research organizations (CROs), and academic research institutions worldwide.
Market Segmentation
The Clinical Trial Biorepository and Archiving Solutions Market is segmented into Service Type, Application, and End User.
By Service Type, the market is categorized into Biorepository Services, Archiving Services, and Integrated Solutions.
By Application, the market is categorized into Drug Discovery, Clinical Trials, and Translational Research.
By End User, the market is categorized into Pharmaceutical & Biotechnology Companies, Contract Research Organizations (CROs), and Academic & Research Institutes.
Growth Driver
The primary growth driver of the Clinical Trial Biorepository and Archiving Solutions Market is the rapid increase in global clinical trial activities. Rising prevalence of chronic diseases, growing investments in drug development, and expansion of biologics and personalized therapies have significantly increased the volume of biological samples and trial data generated. This surge necessitates secure, compliant, and scalable biorepository and archiving solutions to manage complex datasets and biospecimens efficiently. Additionally, stringent regulatory guidelines for data retention and sample traceability further boost market demand.
Market Opportunity
A key market opportunity lies in the adoption of advanced digital archiving and automation technologies. The integration of cloud-based data management systems, AI-enabled sample tracking, and blockchain-based audit trails offers enhanced transparency, operational efficiency, and regulatory compliance. Emerging markets are also presenting lucrative growth opportunities as pharmaceutical companies expand clinical research activities in Asia-Pacific and Latin America, creating increased demand for modern biorepository and archiving infrastructure.
Detailed Segmentation
Clinical Trial Biorepository and Archiving Solutions Market, Segmentation
The Clinical Trial Biorepository and Archiving Solutions Market is segmented on the basis of Service Type, Application, and End User.
Service Type
The Service Type segment is further classified into Biorepository Services, Archiving Services, and Integrated Solutions. Among these, the Biorepository Services sub-segment accounted for the highest market share in 2023. This dominance is attributed to the increasing volume of biospecimens generated during clinical trials and the need for specialized storage conditions. Biorepository services ensure sample integrity through controlled environments, standardized handling procedures, and advanced inventory management systems, making them indispensable for long-term clinical research.
Application
The Application segment is further classified into Drug Discovery, Clinical Trials, and Translational Research. Among these, the Clinical Trials sub-segment held the highest market share in 2023. The growth is driven by the rising number of Phase I–IV trials globally and increasing regulatory scrutiny on sample storage and data archiving. Effective archiving during clinical trials supports compliance, audit readiness, and efficient data retrieval, thereby accelerating drug approval processes.
Some of The Leading/Active Market Players Are-
• Thermo Fisher Scientific Inc. (USA)
• Labcorp Drug Development (USA)
• Charles River Laboratories International, Inc. (USA)
• IQVIA Inc. (USA)
• Azenta Life Sciences (USA)
• Brooks Automation, Inc. (USA)
• Precision for Medicine (USA)
• BioStorage Technologies, Inc. (USA)
• Tecan Group Ltd. (Switzerland)
• Q2 Solutions (USA)
• Cryoport, Inc. (USA)
• Hamilton Company (USA)
• PHC Holdings Corporation (Japan)
• ASKION GmbH (Germany)
• RUCDR Infinite Biologics (USA)
and other active players.
Key Industry Developments
In March 2024, a leading life sciences company expanded its biorepository infrastructure to support large-scale clinical trials.
The expansion included advanced cold storage facilities and digital sample tracking systems, enhancing capacity and compliance for global pharmaceutical clients.
In October 2023, a major CRO partnered with a biorepository service provider to streamline clinical trial sample management.
This collaboration aimed to improve operational efficiency, ensure regulatory compliance, and reduce turnaround times for multinational clinical research programs.
Key Findings of the Study
• Biorepository services dominate the market due to rising biospecimen volumes
• Clinical trials remain the leading application segment
• North America leads the market owing to strong clinical research activity
• Increasing regulatory compliance drives market growth
• Digital archiving trends are shaping future opportunities
More Info:- https://introspectivemarketresearch.com/reports/clinical-trial-biorepository-and-archiving-solutions-market/
About Us
At Introspective Market Research Private Limited, we are a forward-thinking research consulting firm committed to driving growth in the Clinical Trial Biorepository and Archiving Solutions Market. With deep insights, strategic solutions, and holistic research, we empower businesses to achieve success and dominance in the global healthcare and life sciences industry.
📞 Contact Us
Introspective Market Research Pvt. Ltd.
📱 Phone: +91-91753-37569
📧 Email: [email protected]
🌐 Web: www.introspectivemarketresearch.com