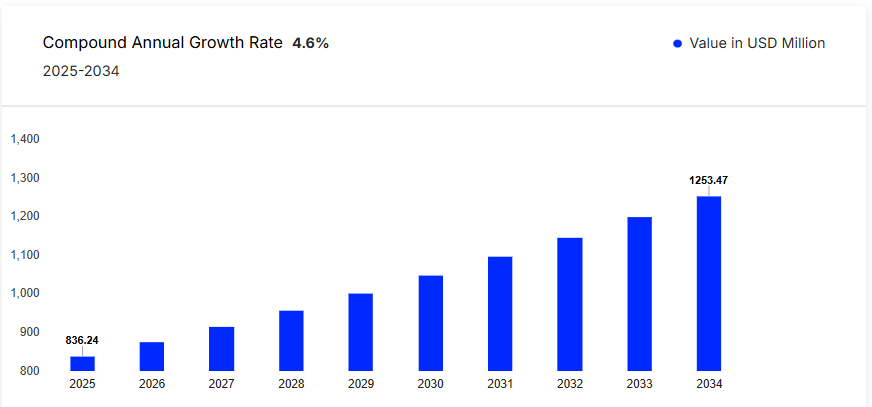
The Chile In-Vitro Diagnostics (IVD) market stands as a pivotal component of the nation's healthcare infrastructure. In-vitro diagnostics encompass a range of medical tests conducted on samples derived from the human body, such as blood, urine, or tissue, to detect diseases, conditions, or infections. These tests are instrumental in disease prevention, monitoring, and management, thereby enhancing patient outcomes and optimizing healthcare delivery.
Understanding the Concept
Core Components
The Chile IVD market integrates several key elements:
-
Clinical Chemistry: Involves the analysis of bodily fluids to detect markers indicative of various conditions, including diabetes and metabolic disorders.
-
Molecular Diagnostics: Utilizes techniques like PCR and next-generation sequencing to identify genetic markers and pathogens, aiding in precise disease diagnosis.
-
Immunoassays: Employs antigen-antibody reactions to detect specific proteins or hormones, crucial in areas like oncology and endocrinology.
-
Hematology: Focuses on blood-related conditions, providing insights into disorders such as anemia and leukemia.
-
Microbiology: Detects infectious agents, facilitating the diagnosis of bacterial, viral, or fungal infections.
-
Point-of-Care Testing (POCT): Enables rapid testing at or near the site of patient care, enhancing immediate decision-making.
Technological Innovations
Advancements in IVD technologies have led to the development of more accurate, rapid, and user-friendly diagnostic tools. The integration of digital health platforms and wearable devices has further revolutionized patient monitoring and management.
The Problem It Solves
Addressing Healthcare Challenges
The Chilean healthcare system faces several challenges:
-
Rising Prevalence of Chronic Diseases: Conditions like diabetes, cardiovascular diseases, and cancer are on the rise, necessitating regular monitoring and early detection.
-
Aging Population: An increasing elderly demographic requires more frequent and diverse diagnostic services.
-
Geographical Barriers: Remote areas often lack immediate access to specialized healthcare facilities.
IVD solutions address these issues by providing accessible, timely, and accurate diagnostic information, enabling early intervention and personalized treatment plans.
Significance
Impact on Stakeholders
-
Patients: Benefit from early disease detection, personalized treatment plans, and improved health outcomes.
-
Healthcare Providers: Gain access to reliable diagnostic tools that inform clinical decisions and enhance patient care.
-
Industry Stakeholders: Experience growth opportunities through the development and distribution of innovative diagnostic products.
The integration of IVD into the healthcare system fosters a more proactive and efficient approach to disease management.
Practical Applications
Real-World Implementation
In Chile, IVD technologies are employed across various settings:
-
Hospitals and Clinics: Utilize advanced diagnostic equipment for comprehensive patient assessments.
-
Diagnostic Laboratories: Specialize in conducting a wide array of tests, supporting both routine and specialized diagnostics.
-
Point-of-Care Settings: Implement rapid testing solutions, particularly in emergency and rural healthcare environments.
These applications have led to improved diagnostic accuracy, reduced turnaround times, and enhanced patient satisfaction.
Future Landscape
Emerging Trends
The Chilean IVD market is poised for growth, influenced by several factors:
-
Technological Advancements: Continued innovation in diagnostic technologies promises more efficient and precise testing methods.
-
Integration with Digital Health: The convergence of IVD with digital health platforms facilitates real-time monitoring and data sharing.
-
Expansion of Point-of-Care Testing: Increasing adoption of POCT solutions aims to bridge healthcare accessibility gaps, especially in underserved regions.
-
Regulatory Support: Favorable policies and regulatory frameworks are expected to encourage the development and adoption of new diagnostic technologies.
Strategic Directions
To capitalize on these trends, stakeholders should focus on:
-
Research and Development: Investing in the creation of innovative diagnostic solutions tailored to local healthcare needs.
-
Collaboration: Engaging with public and private healthcare entities to enhance service delivery and reach.
-
Education and Training: Providing healthcare professionals with the necessary skills to effectively utilize advanced diagnostic tools.