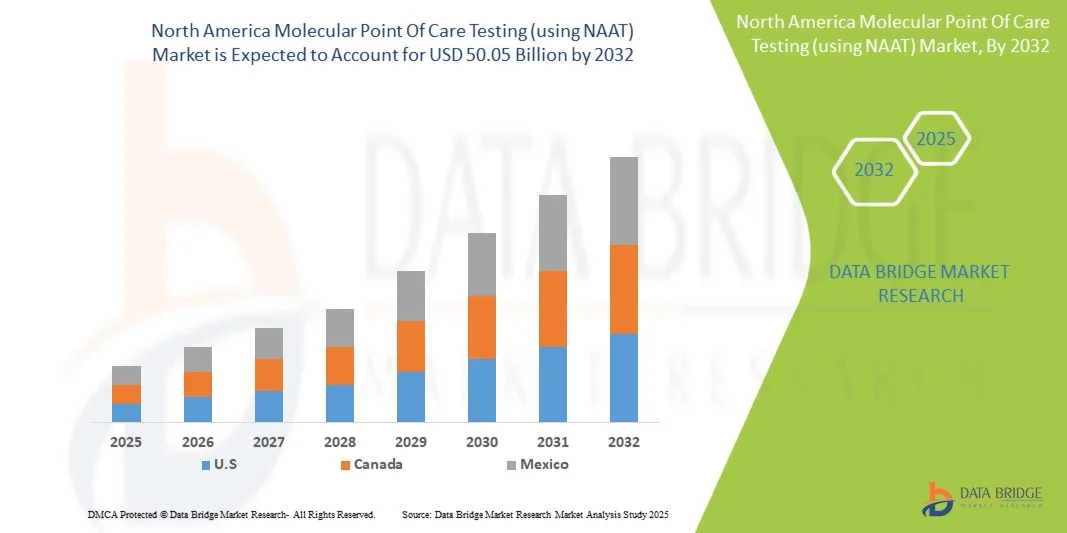
"Executive Summary North America Molecular Point Of Care Testing (using NAAT) Market Trends: Share, Size, and Future Forecast
- The North America molecular point of care testing (using NAAT) market size was valued at USD 20.07 billion in 2024 and is expected to reach USD 50.05 billion by 2032, at a CAGR of 12.1% during the forecast period
Keeping into consideration the customer requirement, North America Molecular Point Of Care Testing (using NAAT) Market research report has been constructed with the professional and comprehensive study. This reliable report comprises of explicit and up to date information about the consumer’s demands, their likings, and their variable preferences about particular product. Market research reports are acquiring huge importance in this speedily transforming market place; hence this market report has been endowed in a way that is anticipated. The world class market report displays several parameters related to North America Molecular Point Of Care Testing (using NAAT) Market industry which are systematically studied by the experts. An influential North America Molecular Point Of Care Testing (using NAAT) Market report is most suitable for business requirements in many ways.
North America Molecular Point Of Care Testing (using NAAT) Market research report is a valuable source of information with which businesses can gain a telescopic view of the current market trends, consumer’s demands and preferences, market situations, opportunities and market status. This market report highlights key market dynamics of sector and encompasses historic data, present market trends, environment, technological innovation, upcoming technologies and the technical progress in the related industry. A lot of hard work has been involved while generating this market research report where no stone is left unturned. Thus, the comprehensive North America Molecular Point Of Care Testing (using NAAT) Market report provides a comprehensive analysis on the study of North America Molecular Point Of Care Testing (using NAAT) Market industry with respect to a number of aspects.
Examine detailed statistics, forecasts, and expert analysis in our North America Molecular Point Of Care Testing (using NAAT) Market report. Download now:
https://www.databridgemarketresearch.com/reports/north-america-molecular-point-of-care-testing-using-naat-market
North America Molecular Point Of Care Testing (using NAAT) Sector Overview
Segments
- Product Type: The North America molecular point of care testing (using NAAT) market can be segmented based on product type into instruments and consumables. Instruments are expected to dominate the market due to the increasing adoption of molecular point of care testing devices to deliver rapid and accurate results.
- Technology: The market can be segmented by technology into polymerase chain reaction (PCR), isothermal nucleic acid amplification technology, and others. PCR technology is anticipated to hold a significant share in the market as it offers high sensitivity and specificity in detecting nucleic acids.
- End-User: Based on end-user, the market is segmented into hospitals, clinics, diagnostic centers, and research laboratories. Hospitals are likely to be the major end-users of molecular point of care testing, attributed to the rising burden of infectious diseases and the need for early diagnostics.
Market Players
- Abbott Laboratories: Abbott is a key player in the North America molecular point of care testing market, offering a wide range of molecular diagnostics solutions, including NAAT-based tests.
- Roche Diagnostics: Roche is another prominent player known for its innovative molecular diagnostic technologies, contributing to the growth of the molecular point of care testing market in North America.
- Cepheid (a Danaher Company): Cepheid, a subsidiary of Danaher, specializes in molecular diagnostics and has a strong presence in the NAAT market with its rapid testing platforms.
- Quidel Corporation: Quidel Corporation is a leading developer of rapid diagnostic testing solutions, including NAAT-based molecular tests for point of care settings.
- BioFire Diagnostics (a bioMérieux Company): BioFire Diagnostics, under the umbrella of bioMérieux, offers molecular testing solutions that play a vital role in point of care testing for rapid and accurate results.
The North America molecular point of care testing (using NAAT) market is witnessing significant growth driven by the increasing demand for rapid and accurate diagnostic solutions. The market segmentation based on product type, technology, and end-user provides insights into the diverse applications of molecular point of care testing. Major market players like Abbott Laboratories, Roche Diagnostics, Cepheid, Quidel Corporation, and BioFire Diagnostics are contributing to market growth through innovative product offerings and strategic collaborations. Overall, the market is poised for expansion in the coming years as the importance of quick and reliable diagnostics continues to rise in healthcare settings.
The North America molecular point of care testing (using NAAT) market is a dynamic landscape driven by advancements in technology and the increasing emphasis on rapid and accurate diagnostics. One emerging trend in the market is the integration of artificial intelligence (AI) and machine learning algorithms into molecular point of care testing devices. These technologies enable faster data analysis, improved accuracy in test results, and enhanced efficiency in diagnosing various infectious diseases. By leveraging AI, healthcare providers can streamline their diagnostic processes, leading to better patient outcomes and reduced healthcare costs. Additionally, the market is witnessing a shift towards miniaturization and portability of molecular testing devices, allowing for point-of-care testing in diverse healthcare settings, including remote locations and resource-limited areas.
Another key aspect shaping the market is the growing focus on decentralized testing solutions. With the decentralization of testing services, healthcare facilities can enhance their capacity to deliver timely diagnoses, particularly in emergency situations or during outbreaks. Decentralized molecular point of care testing also facilitates remote patient monitoring and allows for quick decision-making by healthcare professionals. Moreover, the integration of cloud-based data management systems in molecular testing devices enables real-time data sharing, remote monitoring, and seamless connectivity with electronic health records (EHRs). This connectivity enhances care coordination, facilitates timely intervention, and improves overall healthcare delivery.
Furthermore, the market is witnessing an increasing adoption of multiplex testing assays in molecular point of care testing. Multiplex assays allow for the simultaneous detection of multiple pathogens or genetic markers in a single test, offering significant time and cost savings. These multiplex tests are particularly valuable in outbreak situations, where rapid identification of multiple pathogens is crucial for effective containment and management. Additionally, the development of targeted therapies and personalized medicine approaches is driving the demand for molecular testing solutions that can provide comprehensive genetic information to guide treatment decisions.
In conclusion, the North America molecular point of care testing market is undergoing rapid evolution, fueled by technological innovation, strategic partnerships among market players, and the growing demand for rapid and accurate diagnostic solutions. As the market continues to expand, stakeholders across the healthcare ecosystem must embrace these trends to enhance patient care, improve healthcare outcomes, and drive operational efficiencies. The convergence of AI, decentralized testing solutions, multiplex testing assays, and personalized medicine approaches is reshaping the landscape of molecular point of care testing in North America, opening up new opportunities for innovation and growth.The North America molecular point of care testing market, utilizing NAAT technology, is witnessing robust growth propelled by factors such as increasing demand for rapid and accurate diagnostic solutions. One notable trend shaping the market is the integration of artificial intelligence (AI) and machine learning algorithms into molecular point of care testing devices. This technological integration enhances data analysis speed, boosts test result accuracy, and improves diagnostic efficiency for various infectious diseases. With AI capabilities, healthcare providers can streamline diagnostic processes, leading to better patient outcomes and cost savings in healthcare delivery. Additionally, the market is observing a trend towards miniaturization and portability of testing devices, enabling point-of-care testing in diverse healthcare settings, including remote areas with limited resources.
Decentralized testing solutions are also gaining traction in the market, allowing healthcare facilities to enhance their ability to deliver timely diagnoses, especially in emergency scenarios or outbreaks. Decentralized molecular point of care testing enables remote patient monitoring and facilitates quick decision-making by healthcare professionals. The integration of cloud-based data management systems in testing devices further enhances connectivity with electronic health records (EHRs) and enables real-time data sharing and remote monitoring. This connectivity fosters care coordination, timely interventions, and improved overall healthcare delivery.
Moreover, the rising adoption of multiplex testing assays in molecular point of care testing is a notable trend in the market. Multiplex assays enable the simultaneous detection of multiple pathogens or genetic markers in a single test, offering significant time and cost efficiencies. These assays are particularly valuable in outbreak situations where swift identification of multiple pathogens is crucial for effective containment and management. Additionally, the market is witnessing a surge in demand for molecular testing solutions that provide comprehensive genetic information to guide treatment decisions, driven by the development of targeted therapies and personalized medicine approaches.
As the North America molecular point of care testing market continues to evolve rapidly, driven by technological advancements and strategic collaborations among market players, stakeholders in the healthcare ecosystem must adapt to these trends to enhance patient care, healthcare outcomes, and operational efficiencies. The convergence of AI, decentralized testing solutions, multiplex testing assays, and personalized medicine approaches is reshaping the market landscape, presenting opportunities for innovation and growth in the molecular point of care testing sector in North America.
View company-specific share within the sector
https://www.databridgemarketresearch.com/reports/north-america-molecular-point-of-care-testing-using-naat-market/companies
Strategic Question Sets for In-Depth North America Molecular Point Of Care Testing (using NAAT) Market Analysis
- What is the present valuation of the North America Molecular Point Of Care Testing (using NAAT) Market?
- What is the future growth outlook for the North America Molecular Point Of Care Testing (using NAAT) Market?
- Which are the core market segments detailed in the report?
- Who dominates the competitive landscape of the North America Molecular Point Of Care Testing (using NAAT) Market?
- What are the most recent innovations by players in the North America Molecular Point Of Care Testing (using NAAT) Market?
- Which countries are part of the market coverage in the report?
- Which region is gaining traction rapidly in the North America Molecular Point Of Care Testing (using NAAT) Market?
- Which country is poised to lead in terms of market dominance?
- What area controls the majority share in the North America Molecular Point Of Care Testing (using NAAT) Market?
- Which country is anticipated to see the fastest growth rate?
Browse More Reports:
Global Extruded Polypropylene Foam Market
Global Face Protection Market
Global Factory Automation Sensor Market
Global Farm Product Warehousing and Storage Market
Global Fatty Acid Methyl Ester (FAME) Market
Global Feed Modifiers Market
Global Feed Mycotoxin Binders Market
Global Feed Phytogenics Market
Global Feed Taste Enhancers Market
Global Fertility Supplements Market
Global Fiber Optic Preform Market
Global Finger Print Vehicle Access Market
Global Fish Protein Hydrosylate Market
Global Fish Protein Market
Global Flame Detector Market
Global Livestock Flooring Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- [email protected]
"
