"Global Executive Summary Pathogen Rapid-Seq Screening Devices Market: Size, Share, and Forecast
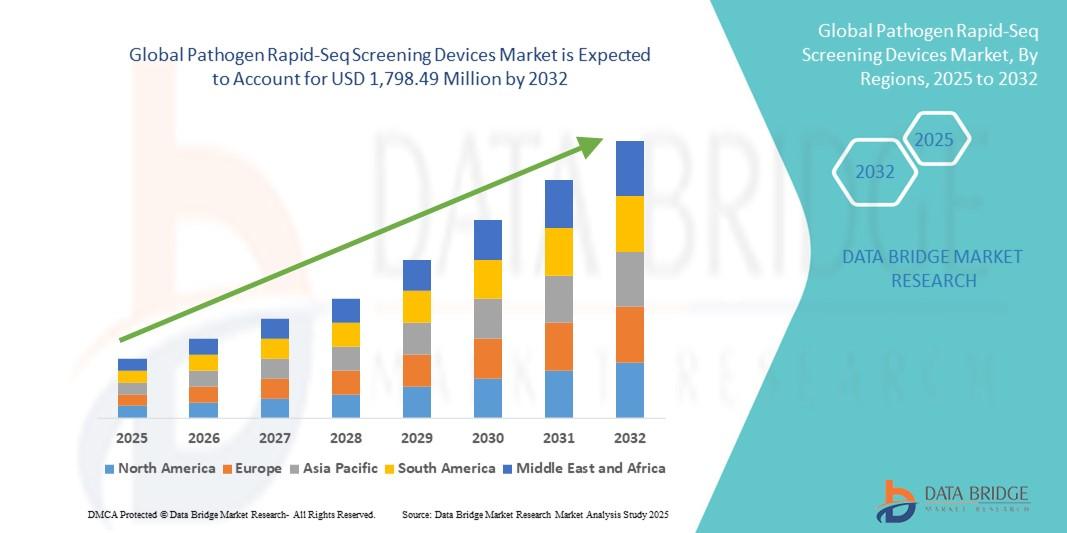
The global pathogen rapid-seq screening devices market size was valued at USD 495.00 million in 2024 and is expected to reach USD 1,798.49 million by 2032, at a CAGR of 17.50% during the forecast period
Pathogen Rapid-Seq Screening Devices Market business report provides data on patterns, improvements, target business sectors, materials, limits, and advancements. With this market report, it becomes possible to gain a holistic view of the market effectively and then also benchmark all the companies in the Pathogen Rapid-Seq Screening Devices Market industry. Moreover, it works to determine the impact of buyers, substitutes, new entrants, competitors, and suppliers on the market. This gives more accurate understanding of the market landscape, issues that may affect the industry in the future, and how to best position specific brands. An influential Pathogen Rapid-Seq Screening Devices Market research document estimates the existing state of the market, market size and market share, revenue generated from the product sale, and necessary changes required in the future products.
The significant Pathogen Rapid-Seq Screening Devices Market research report contains historic data, present market trends, environment, technological innovation, upcoming technologies and the technical progress in the related industry. The research studies involved in this market report helps to estimate several important aspects that includes but are not limited to investment in a rising market, success of a new product, and expansion of market share. Key data and information used while preparing this report has been collected from the consistent sources that range from journals, websites, research papers, case studies, and magazines. Pathogen Rapid-Seq Screening Devices Market report works as a backbone for the growth of Pathogen Rapid-Seq Screening Devices Market industry.
Stay ahead with crucial trends and expert analysis in the latest Pathogen Rapid-Seq Screening Devices Market report.Download now:
https://www.databridgemarketresearch.com/reports/global-pathogen-rapid-seq-screening-devices-market
Pathogen Rapid-Seq Screening Devices Industry Overview
Segments
- Product Type
- Reagents & Kits
- Instruments
- Application
- Disease Diagnosis
- Drug Discovery
- Others
- End-User
- Hospitals
- Diagnostic Laboratories
- Others
Market Players
- Thermo Fisher Scientific Inc.
- QIAGEN
- Abbott
- F. Hoffmann-La Roche Ltd
- Meridian Bioscience, Inc.
- Luminex Corporation
- Quidel Corporation
- ELITechGroup
- GenMark Diagnostics, Inc.
- Hologic, Inc.
The global market for pathogen rapid-seq screening devices is witnessing significant growth due to the rising prevalence of infectious diseases, advancements in molecular diagnostics, and the increasing demand for rapid and accurate diagnostic solutions. The market is segmented by product type, application, and end-user. In terms of product type, reagents & kits and instruments are the main segments driving market growth as they offer efficient and reliable pathogen screening solutions. Regarding application, the devices are used for disease diagnosis, drug discovery, and other purposes, contributing to the market expansion across healthcare and research sectors. The end-user segments include hospitals, diagnostic laboratories, and others, indicating a wide adoption of pathogen rapid-seq screening devices in various healthcare settings.
Key market players such as Thermo Fisher Scientific Inc., QIAGEN, Abbott, F. Hoffmann-La Roche Ltd, Meridian Bioscience, Inc., Luminex Corporation, Quidel Corporation, ELITechGroup, GenMark Diagnostics, Inc., and Hologic, Inc., are actively contributing to the market growth through innovative product launches, strategic partnerships, and acquisitions. These companies are focusing on developing technologically advanced solutions that can provide rapid and accurate results in pathogen screening, thereby strengthening their market position and competitiveness. The market players are also investing in research and development activities to introduce novel products with enhanced features, meeting the evolving demands of the healthcare industry.
DDDDDThe global market for pathogen rapid-seq screening devices continues to evolve with the advancements in molecular diagnostics and the growing focus on efficient disease management solutions. As the healthcare industry faces challenges related to infectious diseases, the adoption of rapid and accurate pathogen screening technologies becomes paramount. In this competitive landscape, market players are striving to differentiate themselves through product innovation and strategic initiatives.
One of the key trends shaping the market is the increasing emphasis on point-of-care testing. Pathogen rapid-seq screening devices that can deliver quick results at the patient's bedside or in remote locations are gaining traction due to their ability to facilitate timely diagnosis and treatment. This trend is reshaping the traditional diagnostics workflow and driving the demand for portable and user-friendly screening devices.
Another significant trend in the market is the integration of artificial intelligence (AI) and machine learning (ML) algorithms into pathogen screening devices. These technologies enhance the accuracy and efficiency of diagnostic processes by analyzing complex data patterns and accelerating decision-making. Market players are exploring AI-powered solutions to improve the sensitivity and specificity of pathogen detection, thereby enhancing clinical outcomes.
Furthermore, the market is witnessing a surge in collaborations and partnerships between industry players and research institutions. These collaborations enable knowledge sharing, technological exchange, and joint product development efforts, fostering innovation in pathogen rapid-seq screening devices. By leveraging each other's expertise and resources, companies can accelerate the commercialization of cutting-edge technologies and expand their market presence.
Moreover, regulatory developments and compliance standards play a critical role in shaping the market dynamics of pathogen rapid-seq screening devices. Adherence to stringent regulatory requirements is essential to ensure the safety, quality, and performance of these devices. Market players are investing in regulatory affairs and quality assurance to navigate the complex regulatory landscape and launch compliant products in different geographical regions.
Overall, the global market for pathogen rapid-seq screening devices is characterized by innovation, collaboration, and regulatory compliance. With a focus on addressing the evolving healthcare challenges associated with infectious diseases, market players are poised to introduce advanced technologies that can revolutionize pathogen screening and enhance patient care outcomes. Staying abreast of emerging trends and leveraging strategic partnerships will be key strategies for companies to maintain a competitive edge in this dynamic market.Pathogen rapid-seq screening devices have become indispensable tools in the healthcare and research sectors, driving significant advancements in the diagnosis and management of infectious diseases. Market players are continuously innovating to meet the increasing demand for rapid and accurate diagnostic solutions, leading to the introduction of cutting-edge technologies in pathogen screening devices. The integration of artificial intelligence and machine learning algorithms is a transformative trend in the market, enhancing the precision and efficiency of diagnostic processes. By leveraging AI-powered solutions, companies can improve the sensitivity and specificity of pathogen detection, ultimately improving clinical outcomes and patient care.
The emphasis on point-of-care testing is reshaping the landscape of pathogen rapid-seq screening devices, with a growing preference for devices that provide immediate results at the patient's bedside or in remote locations. Portable and user-friendly screening devices are gaining traction due to their ability to facilitate timely diagnosis and treatment, addressing the need for efficient disease management solutions. This trend underscores the importance of accessibility and convenience in healthcare settings, driving the demand for devices that can deliver rapid screening capabilities outside traditional laboratory environments.
Collaborations and partnerships between market players and research institutions are fostering innovation in pathogen rapid-seq screening devices, enabling knowledge sharing and joint product development efforts. By leveraging complementary expertise and resources, companies can accelerate the commercialization of advanced technologies and expand their market presence. These collaborative efforts also contribute to the evolution of regulatory standards and quality assurance practices, ensuring the safety and performance of pathogen screening devices in compliance with stringent regulatory requirements.
Regulatory developments play a pivotal role in shaping the market dynamics of pathogen rapid-seq screening devices, with market players investing in regulatory affairs and quality assurance to navigate the complex regulatory landscape. Adherence to regulatory standards is crucial for ensuring the safety, efficacy, and reliability of these devices across different geographical regions. By staying informed about regulatory changes and maintaining compliance with industry standards, companies can demonstrate their commitment to product quality and patient safety, enhancing their credibility in the market.
In conclusion, the global market for pathogen rapid-seq screening devices is driven by innovation, collaboration, and regulatory compliance. Market players are focused on meeting the evolving healthcare challenges associated with infectious diseases through the development of advanced technologies that can revolutionize pathogen screening and improve patient care outcomes. By embracing emerging trends, fostering strategic partnerships, and prioritizing regulatory compliance, companies can stay competitive and continue to drive advancements in pathogen rapid-seq screening technologies.
Access detailed insights into the company’s market position
https://www.databridgemarketresearch.com/reports/global-pathogen-rapid-seq-screening-devices-market/companies
Alternative Research Questions for Global Pathogen Rapid-Seq Screening Devices Market Analysis
- What is the estimated market value of the Pathogen Rapid-Seq Screening Devices Market in 2025?
- What is the forecasted annual growth of the Pathogen Rapid-Seq Screening Devices Market?
- Which industries are key consumers in the Pathogen Rapid-Seq Screening Devices Market segmentation?
- Which companies are currently investing heavily in the Pathogen Rapid-Seq Screening Devices Market?
- What are the most recent product innovations in the Pathogen Rapid-Seq Screening Devices Market?
- What global regions are comprehensively covered in the Pathogen Rapid-Seq Screening Devices Market analysis?
- Which region is expanding the fastest in terms of market penetration?
- What countries are emerging leaders in the Pathogen Rapid-Seq Screening Devices Market?
- What region dominated the market last year?
- What are the top three market trends in the Pathogen Rapid-Seq Screening Devices Market?
Browse More Reports:
Global Healthcare Generative AI Market
Global Ice Lollies Market
Global Neuromuscular Blockade Drugs Market
Global Non-starch Polysaccharide (NSP) Enzyme Market
Global Pet Wearable Market
Global Recreational Cannabis Market
Global Rice Beer Market
Global Scissor Lift Market
Global Surgical Microscopes Market
Global Sweet Spread Market
Global Urinary Incontinence Care Products Market
Global Vinyl Ester Market
Global Loyalty Management Market
Global Baby Food Market
Global Bike Tyre Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- [email protected]
"